Out line of our research
Germ cells are the only cell type in the body that transmit genetic information to the next generation. Somatic cells are merely vehicles for germ cells. Of all germ cell types, primordial germ cells and spermatogonia have mitotic activity. In particular, spermatogonial stem cells (SSCs) are the only germ cell population with self-renewal potential. Although the number of SSCs in testis is very small (0.02-0.03% of all testis germ cells), SSCs are immortal because of their self-renewal activity. One may say that somatic cells evolved in animals to disseminate an individual’s genetic information as widely as possible. Because SSCs are the only germ cells with self-renewal potential and are responsible for maintaining the germline, they probably the most “selfish” cell types in the whole body. However, the continuous replication of SSCs will also produce many genetic mutations, which in some cases causes genetic disorders but in others drives evolution. Yet, despite their biological importance, very little is known about SSCs.
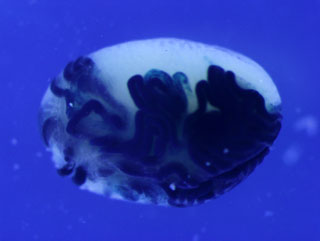
A recipient testis transplanted with donor SSCs
For many years, studies on SSCs were limited mostly to morphological analyses, but in 1994, Dr. Ralph Brinster of the University of Pennsylvania developed a spermatogonial transplantation technique in which dissociated testis cells were microinjected into the seminiferous tubules of infertile recipient mice (Brinster RL, Zimmermann JW, Proc Natl Acad Sci USA 1994;91:11298-11302). These donor SSCs not only generated colonies of germ cells but also resulted in offspring. The development of spermatogonial transplantation technique provided the first functional assay for SSCs and created new opportunities to manipulate these cells for genetic modification of the male germline.
Spermatogonial transplantation has since been used extensively in SSC research, including SSC purification and xenogeneic transplantation. In 2001, retrovirus-mediated gene delivery enabled the establishment of transgenic mice, but the lack of a SSC culture system prevented more sophisticated genetic manipulation. In 2003, our group has established a long-term culture system for SSCs(Kanatsu-Shinohara M.et al., Biol Reprod 2003;69:612-616). The addition of several cytokines, including glial cell line-derived neurotrophic factor, promoted the development of spermatogonial clumps that proliferated for over 2 years without senescence. These cells, which we designated as germline stem (GS) cells and their culture technique have allowed transfection and expansion (by drug selection) of SSC clones in vitro.
GS cells proliferate as spermatogonia in vitro but can differentiate into sperm upon transplantation into the seminiferous tubules. They are phenotypically very stable and maintain a normal karyotype and genomic imprinting patterns for over 2 years without losing fertility (Kanatsu-Shinohara M. et al., Development 2005;132:4155-4163). This is in contrast to embryonic stem (ES) cells, which often acquire chromosomal abnormalities as well as abnormal DNA methylation patterns. The differences between GS and ES cells suggest that postnatal stem cells use a unique mechanism to suppress potential harmful changes.
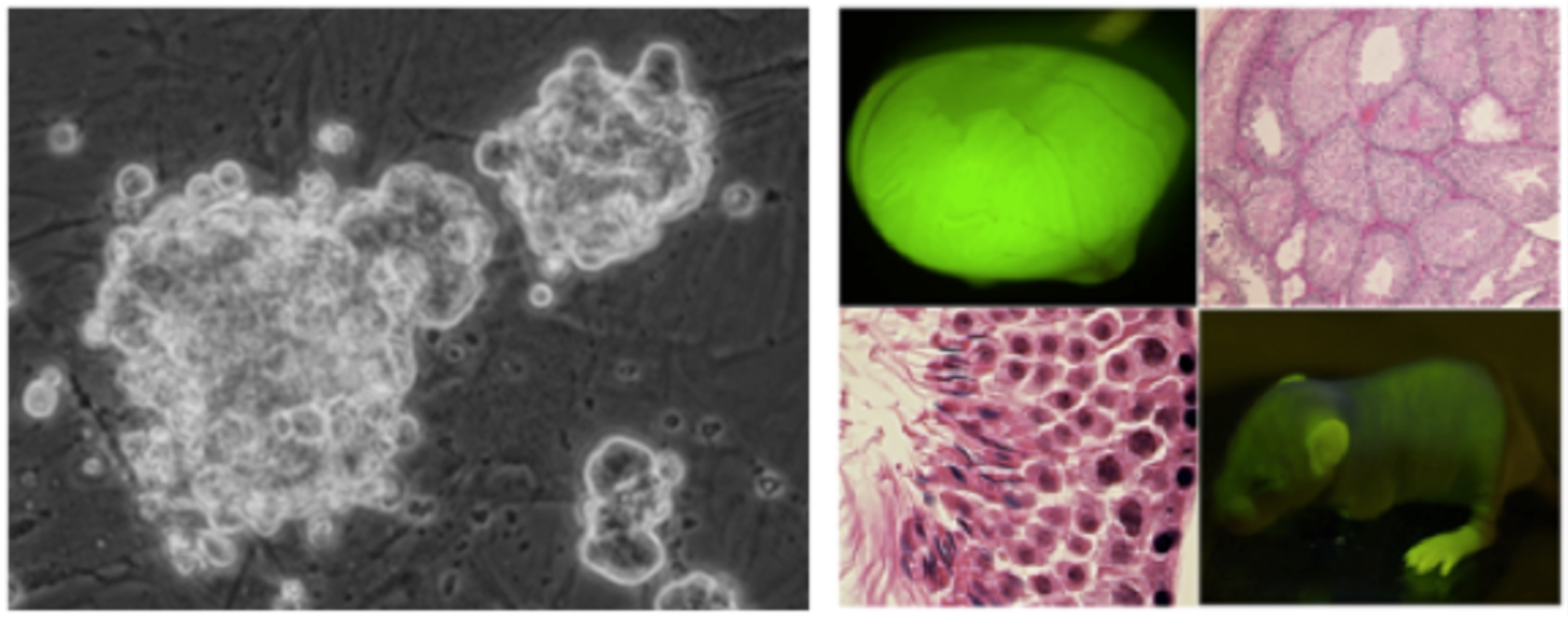
Germline stem (GS) cells cultured on mouse embryonic fibroblasts (left). These cells not only reinitiate spermatogenesis after transplantation into the seminiferous tubules but also produce offspring (right).
Although SSCs are difficult to analyze in vivo due to their small population size, GS cells can be used to collect a large number of SSCs for biochemical and molecular biological analyses. In addition, the cells can be genetically manipulated to produce transgenic and knockout animals, which may be useful for applications in other animal species (Kanatsu-Shinohara M.et al., Proc Natl Acad Sci USA 2006;103:8018-8023). Importantly, human GS cells will be useful for the treatment of male infertility following radio- or chemotherapy for malignancies.
An unexpected property of GS cells is their potential pluripotency. During the course of the gene targeting experiments, we discovered that GS cells transform spontaneously into ES-like multipotent GS cells (mGS) cells (Kanatsu-Shinohara M.et al., Cell 2004;119:1001-1012.). While the frequency of spontaneous mGS cell conversion is very low, we recently found that co-suppression of the p53 and Dmrt1 genes efficiently enhances mGS cell transformation (Takashima S. et al., Genes Dev 2013;27;1949-1958). Together with the finding that deletion of the Max gene in GS cells can induce meiosis (Suzuki A et al., Nat Commun 2016;7:11056), this observation suggests that manipulation of a small number of genes can alter the fate of GS cells dynamically. Although ES cells are the only type of stem cells to produce gametes, the development of GS cells provides unique opportunities for the analysis of SSCs and for genetic modification of the germline.
We are currently working on the following five projects.
- 1) Understanding the self-renewal machinery of SSCs
- 2) Genetic modification of SSCs for producing knockout animals, including in vitro differentiation into sperm
- 3) Analysis of germline transmission patterns from SSCs
- 4) Understanding aging and DNA repair machinery in SSCs
- 5) Regulation of pluripotency in SSCs
Please refer to our previous works in this review (Kanatsu-Shinohara M, Shinohara T, Annu Rev Cell Dev Biol 2013;29;163-187)