Previous research
Fas-induced apoptosis
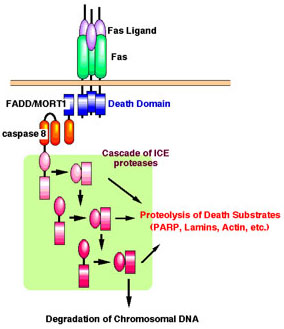
Binding of FasL to Fas induces trimerization of the Fas receptor, which recruits caspase-8 (FLICE/MACH) via an adaptor, FADD/MORT1. The oligomerization of FLICE may result in self-activation of proteolytic activity and trigger the ICE protease cascade. The activated ICE members can cleave various substrates, such as poly(ADP) ribose polymerase (PARP), lamin, rho-GDI, and actin, and cause morphological changes to the cells and nuclei.
DNA fragmentation by CAD during apoptosis
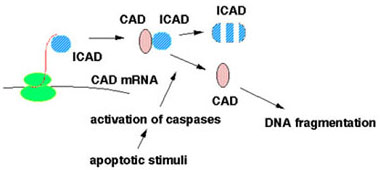
When synthesized, ICAD binds to the nascent chain of CAD to allow its correct folding. ICAD remains complexed with CAD to inhibit the DNase activity of CAD and to mask its nuclear localization signal to keep CAD in the cytoplasm. When apoptotic stimuli activate caspases, ICAD is cleaved and, once released, CAD can enter the nucleus where it degrades chromosomal DNA.
Macrophage-mediated DNA fragmentation
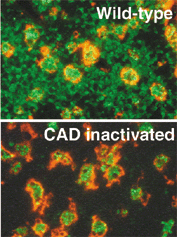
Transgenic mice that ubiquitously express a caspase-resistant form of the ICAD were generated. Thymocytes prepared from the mice were resistant to DNA fragmentation. However, when apoptotic thymocytes from the Tg mice were cocultured with macrophages, the thymocytes were engulfed and their chromosomal DNA underwent fragmentation. The DNA fragmentation that occurs during apoptosis not only can result cell-autonomously from CAD activity but aloso be attributed to a lysosomal acid DNase(s), most likely DNaseII, after the apoptotic cells are engulfed.