PD-1_Project
Member
PD-1(Programmed cell death 1)was identified by our laboratory in 1992 by subtractive-hybridization as a molecule that is upregulated by apoptotic stimuli. To characterize the function of PD-1, we identified its two ligands, analyzed its signal transduction, and examined the pathophysiology of autoimmune diseases which appear
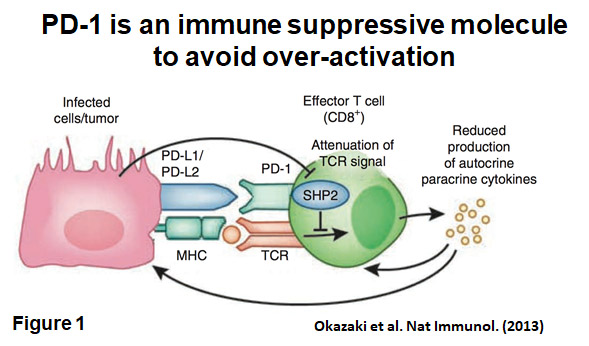
in PD-1 knock out (Pdcd1-/-) mice. The most prominent feature of autoimmune diseases observed in Pdcd1-/- mice is that the symptoms differ between strains. C57BL/6-Pdcd1-/- mice develop lupus-like arthritis and glomerulonephritis. In contrast, BALB/c-Pdcd1-/- mice produce autoantibodies against cardiac troponin I and develop dilated cardiomyopathy. From these observations, it became clear that PD-1 provides negative co-stimulation to lymphocytes, which is critical for the establishment and/or maintenance of self-tolerance (Figure 1).
The recognition of cancer cells as “self” is central to the capacity of tumor cells to escape immune surveillance and subsequently proliferate and undergo metastasis. The development of autoimmune diseases in Pdcd1-/- mice suggests that blockade of the PD-1/PD-L1 pathway would facilitate the recognition of cancer cells as “foreign”. In agreement with this hypothesis, our laboratory demonstrated that tumor cells in mice express PD-L1 to escape from immune surveillance by inhibiting T cell activity. Similarly, cancer patients whose cancer cells highly express PD-L1 experience worse prognosis.
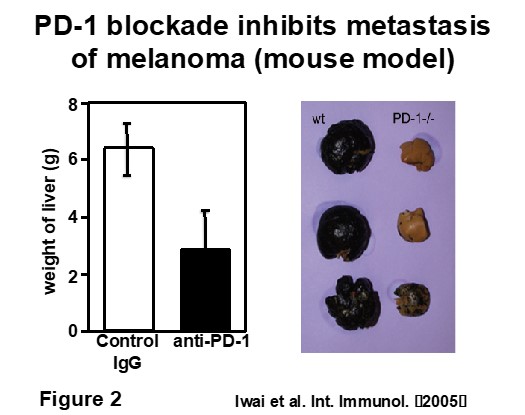
Administration of anti-PD-1 mAb or anti-PD-L1 mAb inhibits tumor growth and metastasis in mouse models (Figure 2). Therefore, we commenced clinical immunotherapy using anti-human PD-1 mAb in collaboration with departments of the Kyoto University Faculty of Medicine and other facilities. Anti-PD-1 mAb was first approved by PMDA as a new cancer drug for melanoma in Japan on July 2014 and since then, anti-PD-1 mAb therapy has become widespread as one of the most effective cancer immunotherapies available. However, a significant proportion of patients remain unresponsive. Therefore, finding predictive biomarkers that discriminate responsive and unresponsive patients and the development of combination therapies which provide relief for these patients is an urgent task. Our laboratory focuses on investigating the mechanism of resistance to the PD-1 blockade therapy and the development of combination therapies using mouse models as well as searching for clinical biomarkers among responsive and unresponsive patients. These days, it has been uncovered that metabolism is closely involved in immune responses. We are working on the above issues related to tumor immunotherapy from the aspect of immune metabolism as well.
References
1. Tanaka K*, Chamoto K*(*equally corresponding), Saeki S, Hatae R, Ikematsu Y, Sakai K, Ando N, Sonomura K, Kojima S, Taketsuna M, Kim YH, Yoshida H, Ozasa H, Sakamori Y, Hirano T, Matsuda F, Hirai T, Nishio K, Sakagami T, Fukushima M, Nakanishi Y, Honjo T, Okamoto I.Combination bezafibrate and nivolumab treatment of patients with advanced non?small cell lung cancer. Sci Transl Med. in press.
2. Matsushima S, Ajiro M, Iida K, Chamoto K, Honjo T, Hagiwara M.
Chemical induction of splice-neoantigens attenuates tumor growth (2022) Sci Transl Med. in press.
3. Al-Habsi M*, Chamoto K*, Matsumoto K*, Nomura N*(*equally contributed), Zhang B, Sugiura Y, Sonomura K, Maharani A, Nakajima Y, Wu Y, Nomura Y, Menzies R, Tajima M, Kitaoka K, Haku Y, Delghandi S, Yurimoto K, Matsuda F, Iwata S, Ogura T, Fagarasan S, Honjo T.
Spermidine activates mitochondrial trifunctional protein and improves antitumor immunity in mice. (2022) Science. 28; 378 (6618): eabj3510. Highlighted by Cancer Discovery:[PubMed]
4. Zhang B, Vogelzang A, Miyajima M, Sugiura Y, Wu Y, Chamoto K, Nakano R, Hatae R, Menzies RJ, Sonomura K, Hojo N, Ogawa T, Kobayashi W, Tsutsui Y, Yamamoto S, Maruya M, Narushima S, Suzuki K, Sugiya H, Murakami K, Hashimoto M, Ueno H, Kobayashi T, Ito K, Hirano T, Shiroguchi K, Matsuda F, Suematsu M, Honjo T, Fagarasan S.
B cell-derived GABA elicits IL-10+ macrophages to limit anti-tumour immunity. (2021) Nature, 599(7885):471-476.
5. Ogishi M, Yang R, Aytekin C, Langlais D, Bourgey M, Khan T, Ali FA, Rahman M, Delmonte OM, Chrabieh M, Zhang P, Gruber C, Pelham SJ, Spaan AN, Rosain J, Lei WT, Drutman S, Hellmann MD, Callahan MK, Adamow M, Wong P, Wolchok JD, Rao G, Ma CS, Nakajima Y, Yaguchi T, Chamoto K, Williams SC, Emile JF, Rozenberg F, Glickman MS, Rapaport F, Kerner G, Allington G, Tezcan I, Cagdas D, Hosnut FO, Dogu F, Ikinciogullari A, Rao VK, Kainulainen L, Beziat V, Bustamante J, Vilarinho S, Lifton RP, Boisson B, Abel L, Bogunovic D, Marr N, Notarangelo LD, Tangye SG, Honjo T, Gros P, Boisson-Dupuis S, Casanova JL.
Inherited PD-1 deficiency underlies tuberculosis and autoimmunity in a child. (2021) Nat Med.27(9):1646-1654.
6. Nakajima Y, Chamoto K, Oura T, and Honjo T.
Critical role of the CD44lowCD62Llow CD8+ T-cell subset in restoring anti-tumor immunity in aged mice. (2021) Proc Natl Acad Sci U S A, 118:e2103730118.
7. Kumar A and Chamoto K.
Immune metabolism in PD-1 blockade-based cancer immunotherapy. (2021) Int Immunol, 33: 17-26, review. [PubMed]
8. Akrami M*, Menzies R*, Chamoto K* (*equally contributed), Miyajima M, Suzuki R, Sato H, Nishii A, Tomura M, Fagarasan S, Honjo T.
Circulation of gut-preactivated naive CD8+ T cells enhances antitumor immunity in B cell-defective mice. (2020) Proc Natl Acad Sci U S A. 117(38):23674-23683. [PubMed]
9. Kumar A*, Chamoto K* (*equally contributed), Chowdhury PS, Honjo T.
Tumors attenuating the mitochondrial activity in T cells escape from PD-1 blockade therapy. (2020) Elife. 9, e52330. [PubMed]
10. Chamoto K, Hatae R, Honjo T.
Current issues and perspectives in PD-1 blockade cancer immunotherapy. (2020) Int J Clin Oncol., 25:790-800. [PubMed]
11. Hatae R*, Chamoto K*(*equally contributed), Kim YH, Sonomura K, Taneishi K, Kawaguchi S, Yoshida H, Ozasa H, Sakamori Y, Akrami M, Fagarasan S, Masuda I, Okuno Y, Matsuda F, Hirai T, Honjo H.
Combination of host immune metabolic biomarkers for the PD-1 blockade cancer immunotherapy (2019) JCI insight, 5, e133501. [PubMed]
12. Goto M*, Chamoto K* (*equally contributed), Higuchi K, Yamashita S, Noda K, Iino T, Miura M, Yamasaki T, Ogawa O, Sonobe M, Date H, Hamanishi J, Mandai M, Tanaka Y, Chikuma S, Hatae R, Muto M, Minamiguchi S, Minato N, Honjo T.
Analytical performance of a new automated chemiluminescent magnetic immunoassays for soluble PD-1, PD-L1, and CTLA-4 in human plasma. (2019) Sci Rep. 9:10144. [PubMed]
13. Mulati K, Hamanishi J, Matsumura N, Chamoto K, Mise N, Abiko K, Baba T, Yamaguchi K, Horikawa N, Murakami R, Taki M, Budiman K, Zeng X, Hosoe Y, Azuma M, Konishi I, Mandai M.
VISTA expressed in tumour cells regulates T cell function. (2019) Br J Cancer. 120:115-127. [PubMed]
14. Chowdhury PS*, Chamoto K* (*equally contributed), Kumar A, Honjo T.
PPAR-induced fatty acid oxidation in T cells increases the number of tumor-reactive CD8+ T cells and facilitates anti-PD-1 therapy. (2018) Cancer Immunol Res, 6: 1375-1387. [PubMed]
15. Chowdhury PS*, Chamoto K* (*equally contributed), Honjo T.
Combination therapy strategies for improving PD-1 blockade efficacy: A new era in cancer immunotherapy.
J Intern Med. 283:110-120 (2018)[PubMed]
16. Miyajima M, Zhang B, Sugiura Y, Sonomura K, Guerrini MM, Tsutsui Y, Maruya M, Vogelzang A, Chamoto K, Honda K, Hikida T, Ito S, Qin H, Sanuki R, Suzuki K, Furukawa T, Ishihama Y, Matsuda F, Suematsu M, Honjo T, Fagarasan S.
Metabolic shift induced by systemic activation of T cells in PD-1-deficient mice perturbs brain monoamines and emotional behavior.
Nat Immunol. 18:1342-1352 (2017)[PubMed]
17. Chamoto K*, Muna Al-Habsi* (*equally contributed), Honjo T.
Role of PD-1 in immunity and diseases.
Curr Top Microbiol Immunol, 198:3757. (2017)[PubMed]
18. Iwai Y*, Hamanishi J*, Chamoto K* (*equally contributed), Honjo T.
Cancer immunotherapies targeting the PD-1 signaling pathway.
J Biomed Sci. 24:26. Review (2017)[PubMed]
19. Chamoto K, Chowdhury PS, Kumar A, Sonomura K, Matsuda F, Fagarasan S, Honjo T.
Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity.
Proc Natl Acad Sci U S A. 114: E761-E770. (2017)[PubMed]
20. Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T.
A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application.
Nat Immunol. 14(12):1212-8 Review (2013) [PubMed]
21. Chikuma S, Suita N, Okazaki IM, Shibayama S, Honjo T.
TRIM28 prevents autoinflammatory T cell development in vivo.
Nat Immunol. 13(6):596-603 (2012) [PubMed]
22. Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S.
Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer.
PNAS. 104(9):3360-5 (2007) [PubMed]
23. Okazaki T, Honjo T.
Rejuvenating exhausted T cells during chronic viral infection.
Cell. 124(3):459-61 (2006) [PubMed]
24. Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T.
PD-1 inhibits antiviral immunity at the effector phase in the liver.
J Exp Med. ;198(1):39-50 (2003). [PubMed]
25. Okazaki, T., Tanaka, Y., Nishio, R., Mitsuiye, T., Mizoguchi, A., Wang, J., Ishida, M., Hiai, H., Matsumori, A., Minato, N., and Honjo, T.
Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice.
Nat Med. 9(12):1477-83 (2003) [PubMed]
26. Iwai, Y., Ishida, M., Tanaka, Y., Okazaki, T., Honjo, T., and Minato N.
Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade.
PNAS 99, 12293-7 (2002) [PubMed]
27. Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T.
Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice.
Science 291(5502):319-22 (2001) [PubMed]